Funariaceae
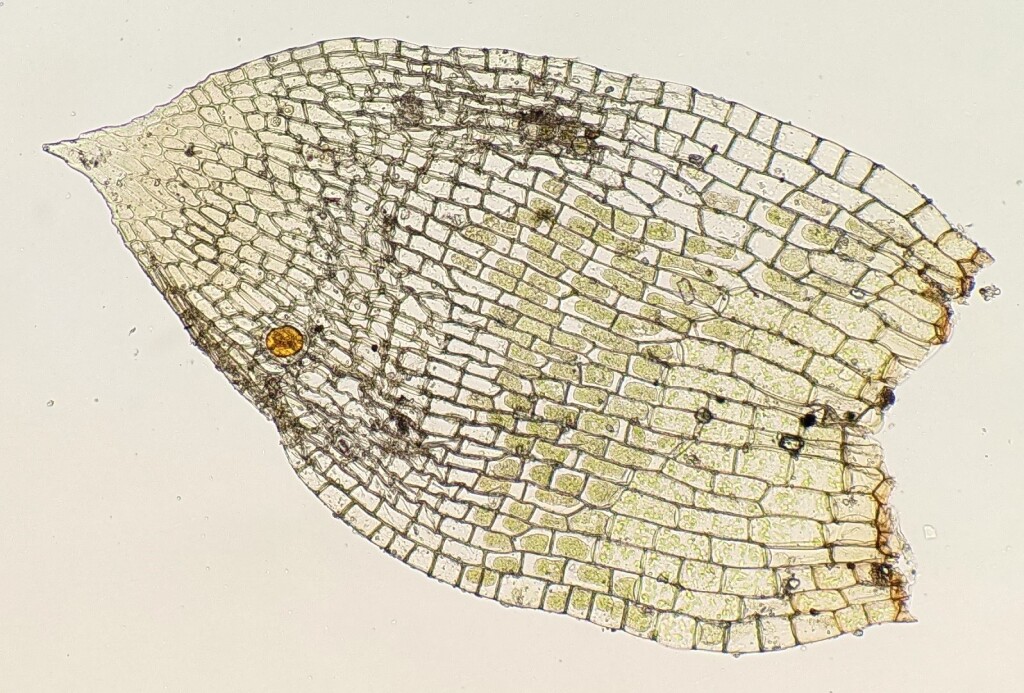
Autoicous, paroicous or rarely synoicous (not in Victoria) or polygamous (not in Victoria). Asexual reproduction rarely by rhizoidal tubers. Minute to small, short-lived plants, gregarious, turves or in open tufts on soil. Stems simple, forked or sparingly branched by innovations, with sparse basal red-brown rhizoids; central strand present. Leaves arranged around stem and facing all directions, usually larger and more crowded toward stem apex, broadly elliptic to obovate, rarely linear (not in Victoria), erect to wide-spreading when moist, scarcely altered, with incurved apices, or usually contorted when dry; apex obtuse, acute or acuminate, sometimes with a hairpoint; costa ending in apical half, percurrent or excurrent, rarely absent or discontinuous; margin entire to serrate, plane to incurved, without a border or sometimes with narrowly oblong cells forming a border; laminal cells large, rhombic to hexagonal or rectangular, smooth, thin-walled, weakly chlorophyllose, usually becoming more elongated toward base; alar cells not or indistinctly differentiated. Acrocarpous. Perigonium with multicellular paraphyses that have globose or pyriform terminal cells. Seta erect or curved, smooth or rarely papillose (not in Victoria). Capsules erect to pendent, globose or pyriform to cupulate, symmetric or asymmetric, immersed or exserted, operculate or cleistocarpous, smooth or striate when dry, usually with a distinct neck, with or without an annulus; stomata present, restricted to neck, consisting of an elongate pore in a single guard cell, immersed or superficial. Calyptra mitrate or cucullate, smooth or papillose (not in Victoria), glabrous, usually strongly rostrate and inflated at base, sometimes pleated. Operculum absent, flat, convex, conic or rostrate. Peristome double, single with only exostome, rudimentary (not in Victoria) or absent; exostome absent, rudimentary (not in Victoria) or 16 entire, straight or sigmoid teeth, sometimes apically fused as a latticed disc; endostome absent, rudimentary or 16 segments, opposite the exostome teeth; cilia absent.
Cosmopolitan, with around 13 genera and 200 species, but revision of genera likely necessary (see Entosthodon profile) which may affect the number of genera recognised in the future (Wilding 2015); four genera and eleven species in Victoria.
The perigonial paraphyses with globose to pyriform terminal cells is a feature unique to the Funariaceae and allows all of its taxa to be distinguished from other mosses (Fife 1985; Liu et al. 2012). Additionally, the taxa that have a double set of peristome have these two sets in a unique arrangement whereby the endostome segments are opposite the exostome teeth and the stomata consist of an elongated pore in a single guard cell, which is a stomatal type only known outside of the Funariaceae in a few Polytrichaceae (Liu et al. 2012). Other features characteristic of many but not all Funariaceae that aids their identification is the often rostrate and inflated calyptra (Fife & Seppelt 2012).
Most of the genera and species in this family are defined by sporophytic features because the sporophyte is highly variable in comparison to the gametophytes that are relatively uniform compared to other moss families (Fife 1985; Fife & Seppelt 2012; Fife 2019). Several Funariaceae genera have been defined by sporophytes that lack several adaptations for aiding spore dispersal, including opercula, peristomes and elongated seta. However, phylogenetic studies of chloroplast (McDaniel et al. 2009; Wilding 2015) or nuclear (McDaniel et al. 2009; Beike et al. 2014; Medina et al. 2019) DNA regions, or all genomic compartments (Liu et al. 2012) to entire genomes (Medina et al. 2018) have shown that a transition from elaborate sporophytes with opercula, peristomes and elevated capsules to reduced sporophytes lacking these characters has occurred recurrently. Several of the genera that have been defined by such homoplasious characters have recently been included in Physcomitrium, including what was previously Physcomitrella readeri (Müll.Hal.) I.G.Stone & G.A.M.Scott in Victoria (Medina et al. 2019). Entosthodon with more elaborate sporophytes, has also been shown to be polyphyletic (Liu et al. 2012; Wilding 2015; Medina et al. 2018, 2019). The phylogenetic positions of three monotypic genera also defined by reduced sporophytes that are known from very few collections remains to be determined and so their status remains uncertain (Medina et al. 2019).
Species in this family are short-lived, but often easily cultured, which has led to some of the species, particularly Physcomitrium patens (Hedw.) Mitt., being used as model organisms for the study of physiology, developmental biology, genome evolution and gene expression (Liu et al. 2012; Fife 2019). Physcomitrium patens was the first non-flowering plant to have its genome completely sequenced and is the bryophyte equivalent to the flowering plant Arabidopsis thaliana (Liu et al. 2012; Fife 2019; Rensing et al. 2020).
 Spinning
SpinningBeike, A.K., von Stackelberg, M., Schallenberg-Rüdinger, Hanke, S.T., Follo, M., Quandt, D., McDaniel, S.F., Reski, R., Tan, B.C.; Rensing, S.A. (2014). Molecular evidence for convergent evolution and allopolyploid speciation within the Physcomitrium-Physcomitrella species complex. BMC Evolutionary Biology 14: 158.
Fife, A.J. (1985). A generic revision of the Funariaceae (Bryophyta: Musci) Part I . Journal of the Hattori Botanical Laboratory 58: 149–196.
Fife, A.J. (2019). Funariaceae, in Smissen, R & Wilton, A.D. (eds), Flora of New Zealand – Mosses. Fascicle 45. Manaaki Whenua Press, Lincoln.
Fife, A.J.; Seppelt, R.D. (2012). Australian Mosses Online. 67. Funariaceae. ABRS, Canberra.
Liu, Y.; Budke, J.M.; Goffinet, B. (2012). Phylogenetic inference rejects sporophyte based classification of the Funariaceae (Bryophyta): Rapid radiation suggests rampant homoplasy in sporophyte evolution. Molecular Phylogenetics and Evolution 62: 130–145.
McDaniel, S.F.; von Stackelberg, M.; Richardt, S.; Quatrano, R.S.; Reski, R.; Rensing, S.A. (2009). The speciation history of the Physcomotrium-Physcomitrella species complex. Evolution 64: 217–231.
Medina, R.; Johnson, M.G.; Liu, Y.; Wickett, N.J.; Shaw, A.J.; Goffinet, B. (2019). Phylogenomic delineation of Physcomitrium (Bryophyta: Funariaceae) based on targeted sequencing of nuclear exons and their flanking regions rejects the retention of Physcomitrella, Physcomitridium and Aphanorrhegma. Journal of Systematics and Evolution 57: 404–417.
Medina, R.; Johnson, M.; Liu, Y.; Wilding, N.; Hedderson, T.A.; Wickett, N.; Goffinet, B. (2018). Evolutionary dynamism in bryophytes: Phylogenomic inferences confirm rapid radiation in the moss family Funariaceae. Molecular Phylogenetics and Evolution 120: 240–247.
Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.-Z.; Bezanilla, M. (2020). The moss Physcomitrium (Physcomitrella) patens: A model organism for non-seed plants. The Plant Cell 32: 1361–1376.
Wilding, N. (2015). Systematics, biogeography and morphological evolution in Entosthodon Schwägr. (Bryopsida, Funariaceae) with a revision of the genus in Africa. PhD Thesis, University of Cape Town.