Rhizogoniaceae
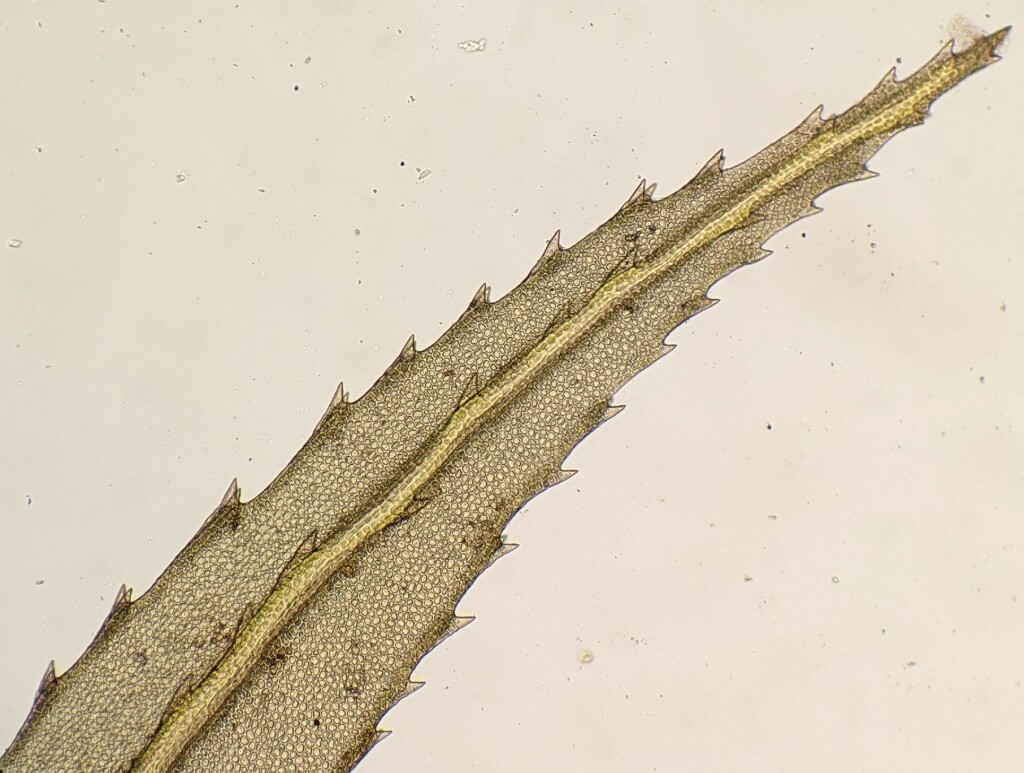
Autoicous or dioicous. Asexual propagules absent. Tufts or turves on trees, tree fern trunks, logs or soil, sometimes with an extensive and persistent protonema. Stems erect or prostrate, simple or branched near stem base or apex (not in Victoria), with brown rhizoids toward base; paraphyllia absent; pseudoparaphyllia absent; central strand present. Leaves distichous, tristichous or inserted in many rows and facing all directions around stem, monomorphic or dimorphic with ventral leaves smaller than lateral leaves, erect-spreading or weakly secund when moist, little altered or twisted when dry, symmetric or asymmetric; apex obtuse, mucronate, acute, or acuminate, without a hairpoint; costa subpercurrent to excurrent; margins mostly with single or paired teeth, less often denticulate or entire, plane, with (not in Victoria) or without a distinct border of elongate cells; laminal cells oblong to hexagonal, usually thick-walled, rarely lax, smooth; alar cells not differentiated. Acrocarpous or pleurocarpous and sporophyte arising from base or lower portions of stem. Capsules erect to pendent, straight or curved, exserted or rarely immersed (not In Victoria), operculate. Calyptra cucullate, smooth or scabrous, glabrous. Operculum conic or rostrate. Peristome double, rarely absent; exostome of 16 undivided teeth; endostome of 16 well developed segments, arising from a basal membrane; cilia present or rudimentary.
Five genera and 24 species, mostly from Australia, Malesia and New Zealand, but extending north to Korea, Japan and China, throughout the Pacific to Tahiti and Hawaii, and also in Madagascar, the Comoros, Reunion, Central and South America, the USA and the Caribbean; four genera and six species in Victoria.
The circumscription of the Rhizogoniaceae presented here differs significantly from the broader Rhizogoniaceae recognised in previous Australian treatments (e.g. Scott & Stone 1976; Gilmore 2006). Genera included in the Rhizogoniaceae in these treatments are placed in separate lineages of combined chloroplast and mitochondrial DNA phylogenies, are non-monophyletic, and as such do not form an appropriate assemblage of genera to be recognised together as a family (Bell et al. 2007). One of these lineages includes Rhizogonium and is equivalent to the Rhizogoniaceae recognised here. The other two lineages that include former Rhizogoniaceae genera comprise a lineage that contains Aulacomnium and another lineage that contains Orthodontium. These lineages now form the Aulacomniaceae and Orthodontiaceae respectively (Bell et al. 2007). Calomnion, which has been recognised in its own family (e.g. Brotherus 1924; Catcheside & Bell 2006), is placed in the lineage with Rhizogonium and so is here included in the Rhizogoniaceae. Many of the Rhizogoniaceae taxa have sporophytes that are borne basally or low on the stem and the gametophyte is tufted and sparingly branched from near the base, however, in Calomnion and the New Zealand Cryptopodium the sporophytes are terminal on the stem and the branching occurs toward the apex in Cryptopodium. The similar habit, complanate arrangement of leaves and lateral leaves between Calomnion and Rhizogonium provide some morphological alignment of Calomnion in Rhizogoniaceae, and Cryptopodium resembles Pyrrhobryum in several features.
 Spinning
SpinningBell, N.E.; Quandt, D.; O’Brien, T.J.; Newton, A.E. (2007). Taxonomy and phylogeny in the earliest diverging pleurocarps: square holes and bifurcating pegs. The Bryologist 110: 533–560.
Brotherus, V.F. (1924). Musci (Laubmoose) II Specieller Teil, in Engler, A. & Prantl, K. (eds.), Die Natürlichen Planzenfamilien. Teil 1. Abt. 3., pp. 277–1246. Engelmann, Leipzig.
Catcheside, D.G.; Bell, G.H. (2006). Calomniaceae, in McCarthy, P.M. (ed.), Flora of Australia. Vol. 51 Mosses 1, pp. 367–368. ABRS, Canberra.
Gilmore, S.R. (2006). Rhizogoniaceae, in McCarthy, P.M. (ed.), Flora of Australia. Vol. 51 Mosses 1, pp. 354–366. ABRS, Canberra.
Scott, G.A.M.; Stone, I.G. (1976). The mosses of Southern Australia. Academic Press, London, New York, San Francisco.