Sphagnaceae
Dioicous or autoicous. Asexual reproduction by fragmentation of branches and stems. Protonemata thallose. Cushions, tufts, mats or turves on wet soil or aquatic in acidic sites. Stems usually erect, simple (not in Victoria) or branched, with a central cylinder of thin-walled parenchymatous cells, merging into a cylinder of thick-walled cortical cells, usually followed by up to 4 layers of thin-walled inflated cells; superficial stem cells fibrillose or not, with pores to the external environment or not; branches usually crowded as a capitulum at apex, usually in fascicles along the stem, spreading and often some pendent and lying against stem in a fascicle; divergent branches sometimes with superficial cells with pores to the external environment on the point of a mamilla (retort cells); rhizoids almost always lacking, present in one species (not in Victoria). Stem and branch leaves arranged around stems and branches, facing all directions, usually markedly different in shape and size; apex apiculate, acute, obtuse or rounded, often erose, without a hairpoint; costa absent; margins usually with a border of narrower, thick-walled hyaline cells; laminal cells unistratose, composed of empty hyaline cells (leucocysts) and more narrow and elongate photosynthetic cells (chlorocysts) intervening hyaline cells; alar cells absent. Stem leaves erect or pendent and appressed to wide-spreading, usually more remote and with a greater width:length than branch leaves, smaller or larger than branch leaves, flat or concave; margins entire or finely fringed away from apex; leucocysts without fibrils and pores to the external environment, or less fibrillose and porose than lycocysts of branch leaves. Branch leaves imbricate, appressed to squarrose when moist or dry, sometimes smaller and narrower on pendent branches than spreading branches, concave; margins entire to dentate, sometimes with marginal row of cells with cell walls partly digested forming a resorption furrow viewed as a ‘C-shaped outline in leaf cross-section; leucocysts usually with one or more pores exposed to external environment on abaxial and adaxial surfaces, sometimes absent adaxially or abaxially (not in Victoria), usually with annular or spiral strengthening fibrils; chlorocysts exposed on both surfaces or enclosed by leucocysts (not in Victoria). Cladocarpous. Seta absent. Capsule erect, symmetric, globose, exserted, elevated on a pseudopodium composed of gametophytic tissue, operculate. Calyptra tiny, membranous, withering with elongation of the pseudopodium. Operculum shallowly convex. Peristome absent.
One genus occurring throughout the world except Antarctica, with differences in species concepts producing drastically differing estimates of the number of species from about 100 species in the most conservative treatment (Andrews 1937) to 300 to 500 species (Eddy 1979; Shaw 2000; Shaw et al. 2003). Most diverse in boreal and subarctic zones of the Northern Hemisphere (Shaw et al. 2010b); four species in Victoria.
Sphagnum is possibly the most widely recognised genus and family of mosses due to their highly distinctive growth form and morphology. Sphagnum often forms distinctive large hummocks, raised among and above other vegetation, in acidic bogs and wetlands. This growth form eventuates by growth at the stem apices and a lack of decay of older supporting tissue below. This is largely due to phenolic compounds in the cell walls that resists decomposition because of its antimicrobial properties (Verhoeven & Toth 1995) in conjunction with low nitrogen content of the Sphagnum and the acidic and oxygen poor environment (Clymo & Hayward 1982).
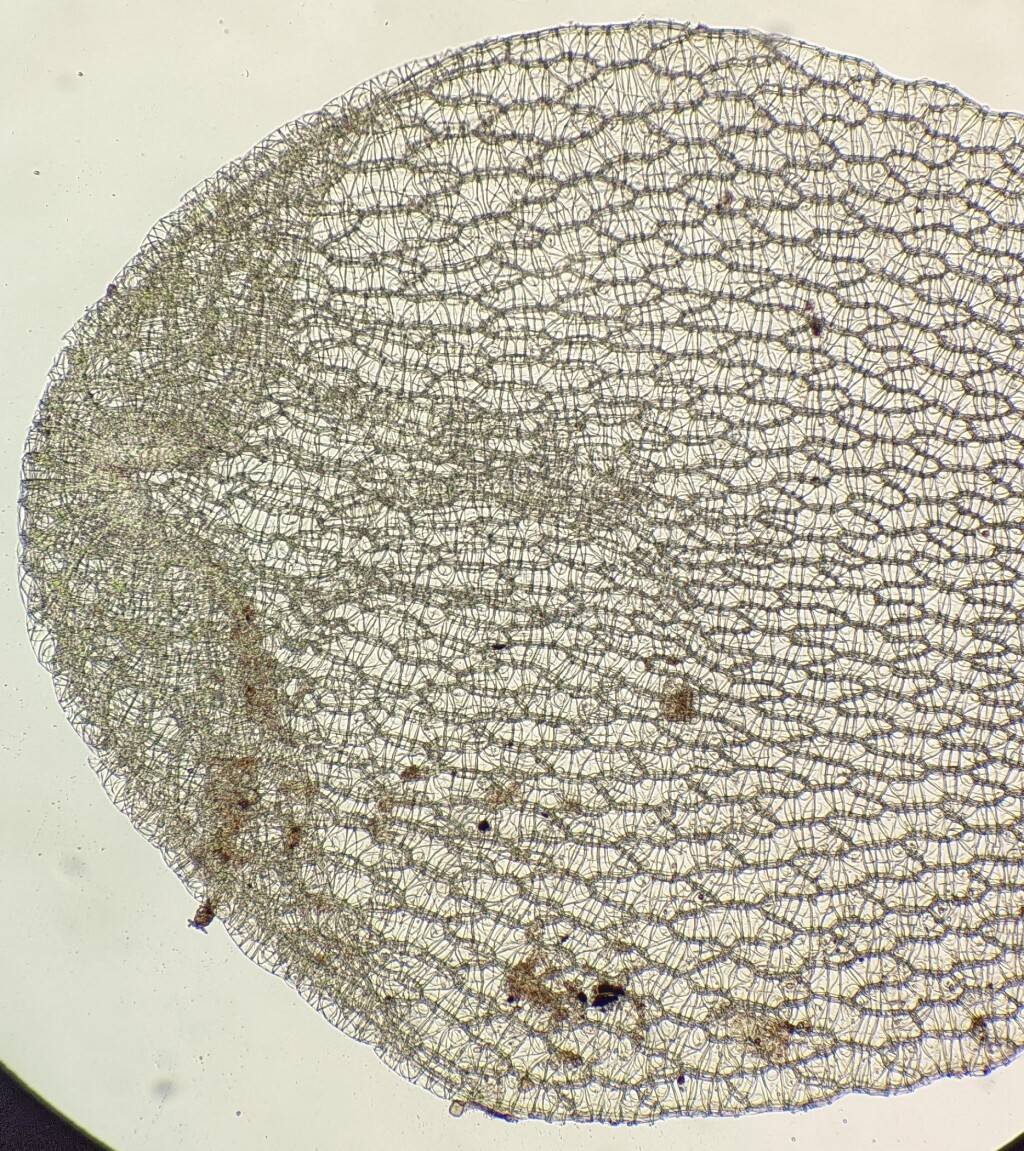
The morphological features of Sphagnum are also highly distinctive and makes Sphagnum easy to distinguish from other moss families. The leaves are composed of large empty hyaline cells with pores exposed to the external environment. When in contact with water, water moves into hyaline cells rapidly through the pores and are capable of retaining this water, which makes the branches and their leaves wet to touch. The sporophyte is also highly distinctive but rarely observed in Victoria. They are black or dark brown spherical capsules elevated on a green stalk (pseudopodium) that is part of the gametophyte rather than the sporophyte. The seta is absent. Capsules dehisce by an apical operculum that explosively ejects upon differential shrinkage of the capsule walls (Duckett et al. 2009).
The Sphagnaceae form a group, classified as the order Sphagnales, with two other families: the Tasmanian and South American Ambuchananiaceae and the Malesian and south-east Asian Flatbergiaceae (Shaw et al. 2010a). Sphagnales is among the earliest diverging lineages of mosses, with the Asian Takakia the only possible lineage to have diverged earlier (Chang & Graham 2011, 2014). Consequently, the Sphagnales are phylogenetically isolated from other mosses, which may partly explain their distinctiveness from other mosses. Despite Sphagnales being one of the oldest lineages of moss, the extant species all appear to have evolved relatively recently based on the similarity of DNA sequences between species (Shaw et al. 2010a, 2010b).
The Sphagnaceae is without doubt the most economically important group of mosses and are used in medicine, horticulture, and indirectly in the production of smoky whisky. The bactericidal properties of Sphagnum conferred largely by phenolic compounds, and the antifungal properties, likely to be conferred largely by coumarins, has led to its use as bandage material for wounds and for treatment of external fungal infections (Podterob & Zubets 2002). The high water holding potential of Sphagnum, imparted by their hyaline cells, makes it a valuable component of potting mixes. Sphagnum is particularly popular in the cultivation of epiphytic species such as orchids that naturally grow among other moss species and species that naturally occur in bogs that require constantly moist conditions. Sphagnum for potting mixes available in Australia is farmed in New Zealand. Peat, which is largely composed of decomposed Sphagnum moss, is also widely used in potting mixes and is burnt to produce smoke to dry malted barley and impart a smoky flavour to smoky whisky.
Sphagnum is also of enormous ecological importance around the world. In the northern hemisphere, peat formed by Sphagnum harbors almost 30 % of the global carbon stored in soil (Gorham 1991). Sphagnum forms a dominant component of bog communities that form habitat for some of Australia’s rarest animals. The critically endangered Southern Corroboree Frog, Pseudophryne corroboree, endemic to the Kosciuszko National Park, lays its eggs in nests of Sphagnum so is dependent on Sphagnum for its survival (Hunter et al. 2009). Sphagnum bog communities have been degraded by previous cattle grazing in alpine areas (Wahren et al. 1999; Whinam et al. 2003). Cattle try to access nearby water sources and damage Sphagnum hummocks through trampling and due to their slow growth do not recover quickly enough to replenish damage done. Alpine and subalpine bogs are now listed as threatened communities under the Flora and Fauna Guarantee Act (1988).
 Spinning
SpinningAndrews, A.L. (1937). Notes on the Warnstorf Sphagnum Herbarium I. Annales Bryologici 9: 3–12.
Chang, Y.; Graham, S.W. (2011). Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set.. American Journal of Botany 98: 839–849.
Chang, Y.; Graham, S.W. (2014). Patterns of clade support across the major lineages of moss phylogeny.. Cladistics 30: 590–606.
Duckett, J.G.; Pressel, S.; P’ng, K.M.Y.; Renzaglia, K.S. (2009). Exploding a myth: the capsule dehiscence mechanism and function of pseudostomata in Sphagnum. New Phytologist 183: 1053–1063.
Eddy, A. (1979). Taxonomy and evolution of Sphagnum, in Clarke, G.C.S. & Duckett, J.G. (eds.), Bryophyte Systematics, pp. 109–121.. Academic Press, London, UK.
Gorham, E. (1991). Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1: 182–195.
Hunter, D.; Osborne, W.; Smith, M.; McDougall, K. (2009). Breeding habitat use and the future management of the critically endangered Southern Corroboree Frog. Ecological Management and Restoration 10.
Podterob, A.P.; Zubets, E.V. (2002). A history of the medicinal uses of plants of the genus Sphagnum. Pharmaceutical Chemistry Journal 36: 192–194.
Shaw, A.J. (2000). Phylogeny of the Sphagnopsida based on nuclear and chloroplast DNA sequences. Bryologist 103: 277–306.
Shaw, A.J.; Cox, C.J.; Boles, S.B. (2003). Polarity of peatmoss (Sphagnum) evolution: who says mosses have no roots?. *American Journal of Botany * 90: 1777–1787.
Shaw, A.J.; Cox, C.J.; Buck, W.R.; Devos, N.; Buchanan, A.M.; Cave, L.; Seppelt, R.; Shaw, B.; Larraín, J.; Andrus, R.; Greilhuber, J.; Temsch, E.M. (2010a). Newly resolved relationships in an early land plant lineage: Bryophyta class Sphagnopsida (peat mosses). *American Journal of Botany * 97: 1511–1531.
Shaw, A.J.; Devos, N.; Cox, C.J.; Boles, S.B.; Shaw, B.; Buchanan, A.M.; Cave, L.; Seppelt, R. (2010b). Peatmoss (Sphagnum) diversification associated with Miocene Northern Hemisphere climatic cooling?. * Molecular Phylogenetics and Evolution* 55: 1139–1145.
Verhoeven, J.T.A.; Toth, E. (1995). Decomposition of Carex and Sphagnum litter in fens: effect of litter quality and inhibition by living tissue homogenates. Soil Biology and Biochemistry 27: 271–275.
Wahren, C.-H.A.; Williams, R.J.; Papst, W.A. (1999). Alpine and subalpine wetland vegetation on the Bogong High Plains, south-eastern Australia. Australian Journal of Botany 47: 165–188.
Whiniam, J.; Chilcott, N.M.; Morgan, J.W. (2003). Floristic composition and environmental relationships of Sphagnum-dominated communities in Victoria. Cunninghamia 8: 162–174.