Amblystegiaceae
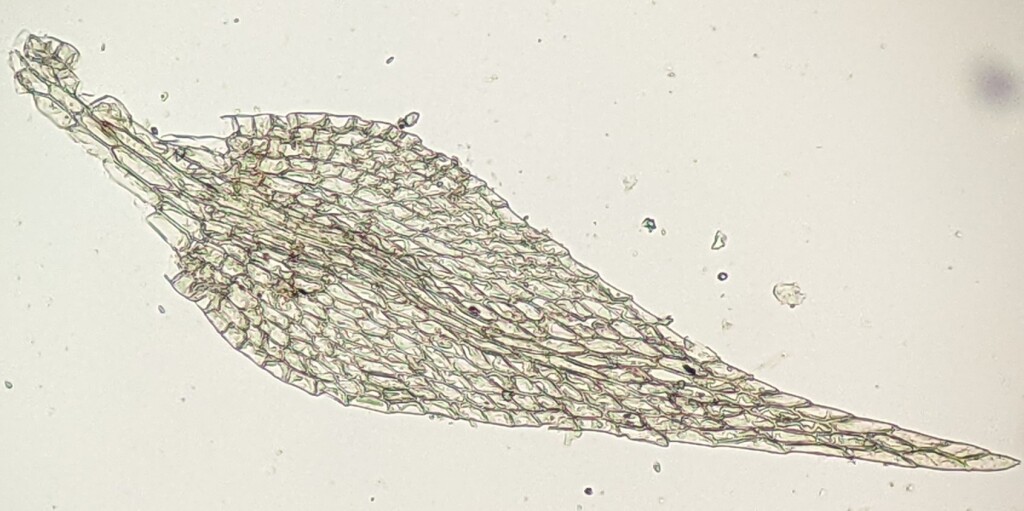
Autoicous, synoicous (not in Victoria) or dioicous. Dense mats or wefts on soil, rocks and logs, often aquatic. Stems creeping, free-floating, or ascendant, irregularly to sparingly or pinnately branched, rarely simple, glabrous or with rhizoids in fascicles or spread along stem or on abaxial costa insertion, rarely forming tomentum; paraphyllia absent or present and filamentous or narrowly lanceolate; pseudoparaphyllia present, foliose; central strand present or rarely absent (not in Victoria). Leaves arranged around stem and facing all directions or complanate, monomorphic or branch leaves smaller than stem leaves, erect- to squarrose when moist, scarcely altered, twisted, or becoming more erect or appressed when dry, straight to circinate, not or sometimes (not in Victoria) decurrent; apex acute or acuminate, or rarely obtuse (not in Victoria) or rounded (not in Victoria); costa single, terminating in basal or apical half of leaf, percurrent or excurrent, short and double, or rarely absent (not in Victoria); margin entire, denticulate or serrulate, plane, recurved, or involute, without a border or rarely with a distinct border of more elongated cells (not in Victoria); laminal cells linear, rhomboid (not in Victoria) or long-hexagonal, smooth or prorate; alar cells not differentiated or differentiated and elliptic or quadrate to rectangular, enlarged or inflated and hyaline. Pleurocarpous. Capsules inclined to horizontal, rarely erect (not in Victoria), oblong to cylindric, curved or rarely straight (not in Victoria), exserted, operculate, with or without an annulus. Calyptra cucullate, smooth, glabrous. Operculum conic, apiculate, or rarely rostrate (not in Victoria). Peristome double and alternate; exostome of 16 entire teeth; endostome of 16 segments, with a high or rarely low (not in Victoria) basal membrane; cilia present or rarely absent (not in Victoria).
Worldwide with around 19 genera and 80 species; five genera and eight species in Victoria.
Phylogenetic analyses of combined chloroplast and nuclear DNA sequences revealed the need to revise the circumscription of the traditional Amblystegiaceae that was defined by features that are highly correlated with environment rather than genetic lineages (Vanderpoorten et al. 2002a). The traditional Amblystegiaceae was shown to comprise two groups separated in DNA phylogenies by other families (Vanderpoorten et al. 2002a, b; Sheng et al. 2020). One of these groups has been recognised as a more narrowly circumscribed Amblystegiaceae (Vanderpoorten et al. 2002b) and this narrower circumscription of the Amblystegiaceae is followed here. In addition to traditional Amblystegiaceae genera this new circumscription of Amblystegiaceae includes the Vittiaceae (Vanderpoorten et al. 2003) and Anacamptodon, which has been included in the Fabroniaceae and has several sporophytic characters that differ from the remaining Amblystegiaceae that are correlated with an epiphytic occurrence (rather than marsh typical of other Amblystegiaceae), such as erect straight capsules, and a peristome with reflexed exostome teeth when dry and a low basal membrane to the endostome (Vanderpoorten et al. 2002a, 2002b). The other group has been recognised as the Calliergonaceae, which in Victoria includes the genera Sarmentypnum and Warnstorfia (Vanderpoorten et al. 2002b). Apparently this new narrowly circumscribed Amblystegiaceae is without morphological characters that are shared by all taxa and can distinguish this family from related families (Vanderpoorten et al. 2002b). However, all Victorian species possess the following combination of characters: a single or rarely double costa, a conic operculum, curved cylindric capsule that is inclined to horizontal and has a ‘perfect’ peristome consisting of well-formed exostome teeth and distinct endostome segments with a high basal membrane and cilia.
The placement of the genus Sanionia requires further investigation. It has been traditionally included in the Amblystegiaceae based on morphology, but conflicting placements are recovered in phylogenies of DNA sequences. Sanionia does not appear to belong to either Amblystegiaceae or the Calliergonaceae in phylogenies incorporating a large number of Hypnalean and traditional Amblystegiaceae species but based on few DNA regions (Vanderpoorten et al. 2002a, 2003), however, in phylogenies of entire chloroplast genomes of a few hypnalean species including some Amblystegiaceae and Calliergonaceae species, Sanionia groups with the Amblystegiaceae (Sheng et al. 2020). For this reason, Sanionia is retained within the Amblystegiaceae here.
 Spinning
SpinningSheng, W.; Yue, X.-R.; Li, N.; Liu, Y.; Wu, Y.-H. (2020). Comparison of eight complete plastid genomes from three moss families Amblystegiaceae, Calliergonaceae and Pylaisiaceae. Mitochondrial DNA Part B 5: 3091–3093.
Vanderpoorten, A.; Goffinet, B.; Hedenäs, L.; Cox, C.J.; Shaw, A.J. (2003). A taxonomic reassessment of the Vittiaceae (Hypnales, Bryopsida): evidence from phylogenetic analyses of combined chloroplast and nuclear sequence data. Plant Systematics and Evolution 241: 1–12.
Vanderpoorten, A.; Hedenäs, L.; Cox, C.J.; Shaw, A.J. (2002a). Phylogeny and morphological evolution of the Amblystegiaceae (Bryopsida). Molecular Phylogenetics and Evolution 23: 1–21.
Vanderpoorten, A.; Hedenäs, L.; Cox, C.J.; Shaw, A.J. (2002b). Circumscription, classification and taxonomy of Amblystegiaceae (Bryopsida) inferred from nuclear and chloroplast DNA sequence data and morphology. Taxon 51: 115–122.