Pottiaceae
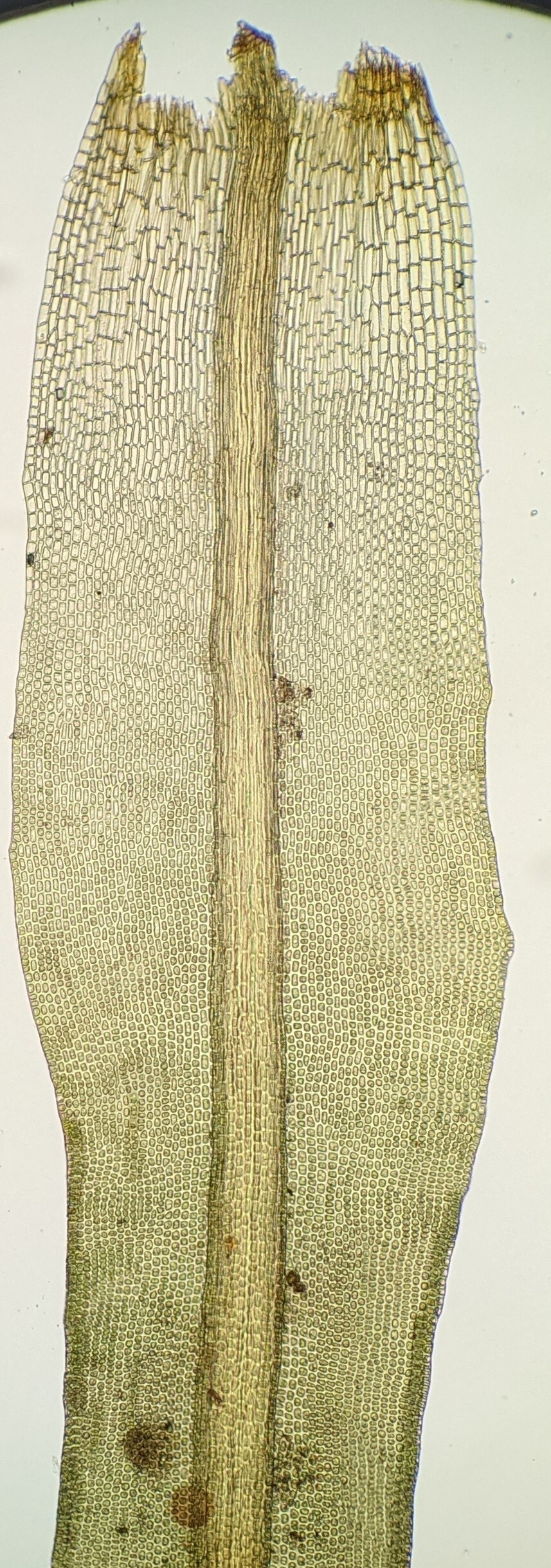
Dioicous, autoicous, paroicous, or rarely rhizautoicous or synoicous (not in Victoria). Asexual reproduction by gemmae borne on stalks in leaf axils or on leaves, by rhizoidal tubers, or rarely by fragile leaves or stems. Turves, mats, tufts, cushions or scattered individuals on soil, rocks or trees, rarely semi-aquatic, sometimes attached to a persistent protonema. Stem erect, sometimes minute, simple or sparingly and irregularly branched, glabrous to tomentose throughout; central strand present, rarely absent. Leaves arranged around stem and facing all directions, or arranged in three rows, monomorphic or becoming longer toward stem apex, erect- to wide-spreading or squarrose when moist, usually contorted and corkscrewed around stem or contorted and spreading when dry, occasionally remaining straight and becoming appressed when dry; base occasionally sheathing stem; apex rounded or obtuse to more commonly narrowly acute or acuminate, with or without a hairpoint; costa subpercurrent to long-excurrent, rarely interrupted or absent (not in Victoria), sometimes with adaxial photosynthetic filaments or adaxial or abaxial (not in Victoria) longitudinally oriented plates of cells (lamellae), sometimes broad and occupying greater than 1/3 of basal leaf width; margin entire, denticulate, or rarely serrate or dentate in the apical half, involute, incurved, plane, revolute or recurved, occasionally with a border of more elongate, thicker-walled or less papillose cells, sometimes border intramarginal; laminal cells subquadrate, hexagonal or rarely short-rectangular or rhomboid in apical half, usually becoming clear, larger and rectangular toward base, papillose and often obscuring cell outline, or rarely smooth or prorate in apical half, smooth or less papillose toward base; alar cells not differentiated. Acrocarpous or occasionally cladocarpous. Seta often twisted when mature. Capsules erect or inclined, straight or curved, immersed, emergent, or exserted, operculate or occasionally cleistocarpous. Calyptra cucullate or occasionally mitrate, smooth or rarely papillose, glabrous. Operculum short-conic to rostrate. Peristome usually a single series of 16 or 32 entire teeth or filaments, erect, or spirally twisted anticlockwise or rarely clockwise (not in Victoria), or occasionally absent.
This is the largest family of moss, with around 98 genera and 1420 species, and has a worldwide distribution; 27 genera and 65 species in Victoria.
Ephemerum is included here in the Pottiaceae following Goffinet & Buck (2004), based on its derivation from Pottiaceae ancestors according to phylogenies of chloroplast DNA sequences (Werner et al. 2004, 2007; Cox et al. 2010; Inoue & Tsubota 2016). Prior to molecular phylogenetics, Ephemerum was placed in its own family as it differs from other Pottiaceae by its large, elongate and smooth laminal cells in the apical half (Zander 2006).
Generic delimitation in the Pottiaceae is highly problematic. Varying taxonomic importance has been placed on morphological features that vary among Pottiaceae, with some features originally considered diagnostic of some genera eventually shown to be plesiomorphic or have arisen independently multiple times in the family, including simplification of sporophyte from more complex sporophytes (Zander 1993; Werner et al. 2004; Cano et al. 2021). To overcome these issues Zander (1993) provided a revision of generic limits within the family which was based on a phylogeny of morphological characters. Generic limits and identification keys were largely based on gametophytic features which was practical for identification as many taxa in this family rarely produce sporophytes (Zander 2007; Inoue & Tsubota 2016). However, classifications like Zander (1993) that were based solely on morphology recognised genera that comprise more than one lineage separated by other genera in phylogenies of chloroplast and nuclear DNA sequences (e.g. Werner et al. 2002, 2004, 2005; Grundmann et al. 2006; Cano et al. 2009, 2021; Kučera et al. 2013; Jiménez et al. 2021). Phylogenetic reconstruction of the family using DNA sequences is still largely incomplete, with the vast majority of taxa not yet sampled, and of those that have, their relationships not strongly supported due to too few DNA regions sequenced in analyses (Stech et al. 2012). As a better knowledge of relationships within the family is gained through further combined molecular and morphological study using a greater number of DNA regions and a greater coverage of taxa, generic circumscriptions are likely to change to match true evolutionary lineages and this will also likely affect the names of Victorian taxa.
In the vast majority of cases the Pottiaceae are distinguished by strongly differentiated costal anatomy and complexly papillose laminal cells in the apical half of the leaf and many species have a twisted peristome and inhabit dry and exposed habitats (Zander 2007). The Pottiaceae are usually the most diverse plant group in dry exposed soils and as such are ecologically important as a major component of biological soil crusts (Eldridge & Tozer 1996; Milne et al. 2006; Seppelt et al. 2016). These soil crusts have a pivotal role in the maintenance of soil by binding together soil at the surface, minimising erosion by wind or high energy rainfall drops, and by aiding its further development through accumulating windblown sediment, forming a habitat for invertebrates involved in nutrient cycling, and contributing directly to organic matter in the soil (see Eldridge & Tozer 1996).
Identification keys to Pottiaceae are usually heavily reliant on technical characters associated with costal and stem anatomy, and colour of leaf cell walls upon reaction to potassium hydroxide (e.g. Zander 1993, 2007), which are not easily assessed by the novice. While taxonomically informative, for ease of identification for a broad audience of users such technical features have been largely avoided in the key to genera provided here.
 Spinning
SpinningCano, M.J.; Jiménez, J.A.; Gallego, M.T.; Guerra, J. (2021). A molecular approach to the phylogeny of the moss genus Pseudocrossidium (Pottiaceae, Bryopsida) and its taxonomic implications . Journal of Systematics and Evolution.
Cano, M.J.; Jiménez, J.F.; Gallego, M.T; Jiménez, J.A; Guerra, J. (2009). Phylogenetic relationships in the genus Hennediella (Pottiaceae, Bryophyta) inferred from nrITS sequence data. Plant Systematics and Evolution 281: 209–216.
Cox, C.J.; Goffinet, B.; Wickett, N.J.; Boles, S.B.; Shaw, A.J. (2010). Moss diversity: A molecular phylogenetic analysis of genera. Phytotaxa 9: 175–195.
Eldridge, D.J.; Tozer, M.E. (1996). Distribution and floristics of Bryophytes in soil crusts in semi-arid and arid eastern Australia. Australian Journal of Botany 44: 223–247.
Goffinet, B.; Buck, W. R. (2004). Systematics of the Bryophyta (Mosses): from Molecules to a Revised Classification. Monographs in Systematic Botany from the Missouri Botanical Garden 98: 205–239.
Grundmann, M.; Schneider, H.; Russell, S.J.; Vogel, J.C. (2006). Phylogenetic relationships of the moss genus Pleurochaete Linb. (Bryales: Pottiaceae) based on chloroplast and nuclear genomic markers. Organisms, Diversity & Evolution 6: 33–45.
Inoue, Y.; Tsubota, H. (2016). Systematics of the family Pottiaceae (Bryophyta) with special reference to the familial and subfamilial circumscriptions. Hikobia 17: 117–129.
Jiménez, J.A.; Cano, M.J.; Guerra, J. (2021). A multilocus phylogeny of the moss genus Didymodon and allied genera (Pottiaceae): Generic delimitations and their implications for systematics. Journal of Systematics and Evolution.
Kučera, J.; Košnar, J.; Werner, O. (2013). Partial generic revision of Barbula (Musci: Pottiaceae): Re-establishment of Hydrogonium and Streblotrichum, and the new genus Gymnobarbula. Taxon 62: 21–39.
Milne, J.; Short, M.; Beckmann, K. (2006). A preliminary study of bryophytes and invertebrates of soil crusts in the Little Desert National Park and surrounds. The Victorian Naturalist 123: 195–203.
Seppelt, R.D.; Downing, A.J.; Deane-Coe, K.K.; Zhang, Y.; Zhang, J. (2016). Bryophytes within biological soil crusts, in Weber, B., Büdel, B. & Belnap, J. (eds), Biological soil crusts: An organizing principle in drylands, pp. 101–120. Springer, New York.
Stech, M.; McDaniel, S.F.; Hernández-Maqueda, R.; Ros, R.M.; Werner, O.; Muñoz, J.; Quandt, D. (2012). Phylogeny of haplolepideous mosses – challenges and perspectives. Journal of Bryology 34: 173–186.
Werner, O.; Ros, R.M.; Cano, M.J.; Guerra, J. (2002). Tortula and some related genera (Pottiaceae, Musci): phylogenetic relationships based on chloroplast rps4 sequences. *Plant Systematics and Evolution * 235: 197–207.
Werner, O.; Ros, R.M.; Cano, M.J.; Guerra, J. (2004). Molecular phylogeny of Pottiaceae (Musci) based on chloroplast rps4 sequence data. Plant Systematics and Evolution 243: 147–164.
Werner, O.; Ros, R.M.; Goffinet, B. (2007). A reconsideration of the systematic position of Goniomitrium (Funariaceae) based on chloroplast sequence markers. The Bryologist 110: 108–114.
Werner, O.; Ros, R.M.; Grundmann, M. (2005). Molecular phylogeny of Trichostomoideae (Pottiaceae, Bryophyta) based on nrITS sequence data. Taxon 54: 361–368.
Zander, R.H. (1993). Genera of the Pottiaceae: Mosses of harsh environments. Bulletin of the Buffalo Society of Natural Sciences 32: 1–378.
Zander, R.H. (2007). Potiaceae, in Flora of North America Editorial Committee (eds), Flora of North America, vol. 27: Bryophyta, part 1, pp. 1–734. Oxford University Press, Oxford.